
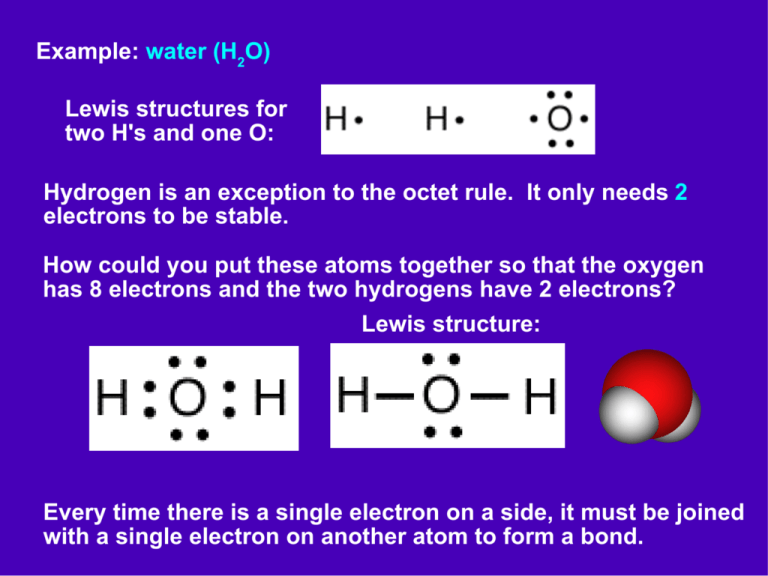
Lewis Dot Diagram For H2o Free Diagram For Student
Steps of drawing H2O2 lewis structure Step 1: Find the total valence electrons in H2O2 molecule. In order to find the total valence electrons in H2O2 molecule, first of all you should know the valence electrons present in hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I'll tell you how you can easily.
Draw lewis struture of H2O2(hydrogen peroxide)
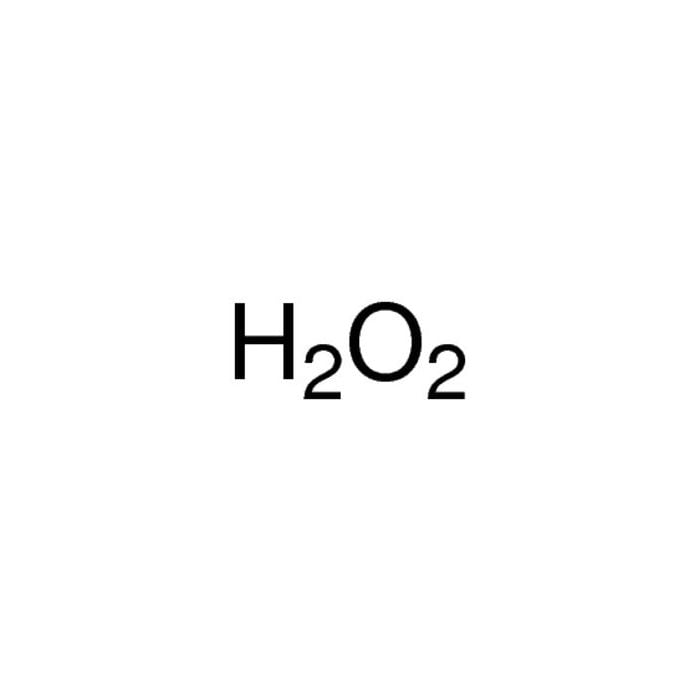
Hydrogen peroxide is a chemical compound with the formula H 2 O 2.In its pure form, it is a very pale blue liquid that is slightly more viscous than water.It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%-6% by weight) in water for consumer use, and in higher concentrations for industrial use.Concentrated hydrogen peroxide, or "high-test peroxide.

H2O2 Lewis Structure (Hydrogen Peroxide) YouTube
O. 2. ) Lewis Structure. Lewis structure of Hydrogen peroxide (H 2 O 2) contains two O-H bonds and one O-O bond. Also, there are two lone pairs on each oxygen atom. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2. Each step of drawing lewis structure of H 2 O 2 is explained.

hydrogen peroxide lewis dot structure
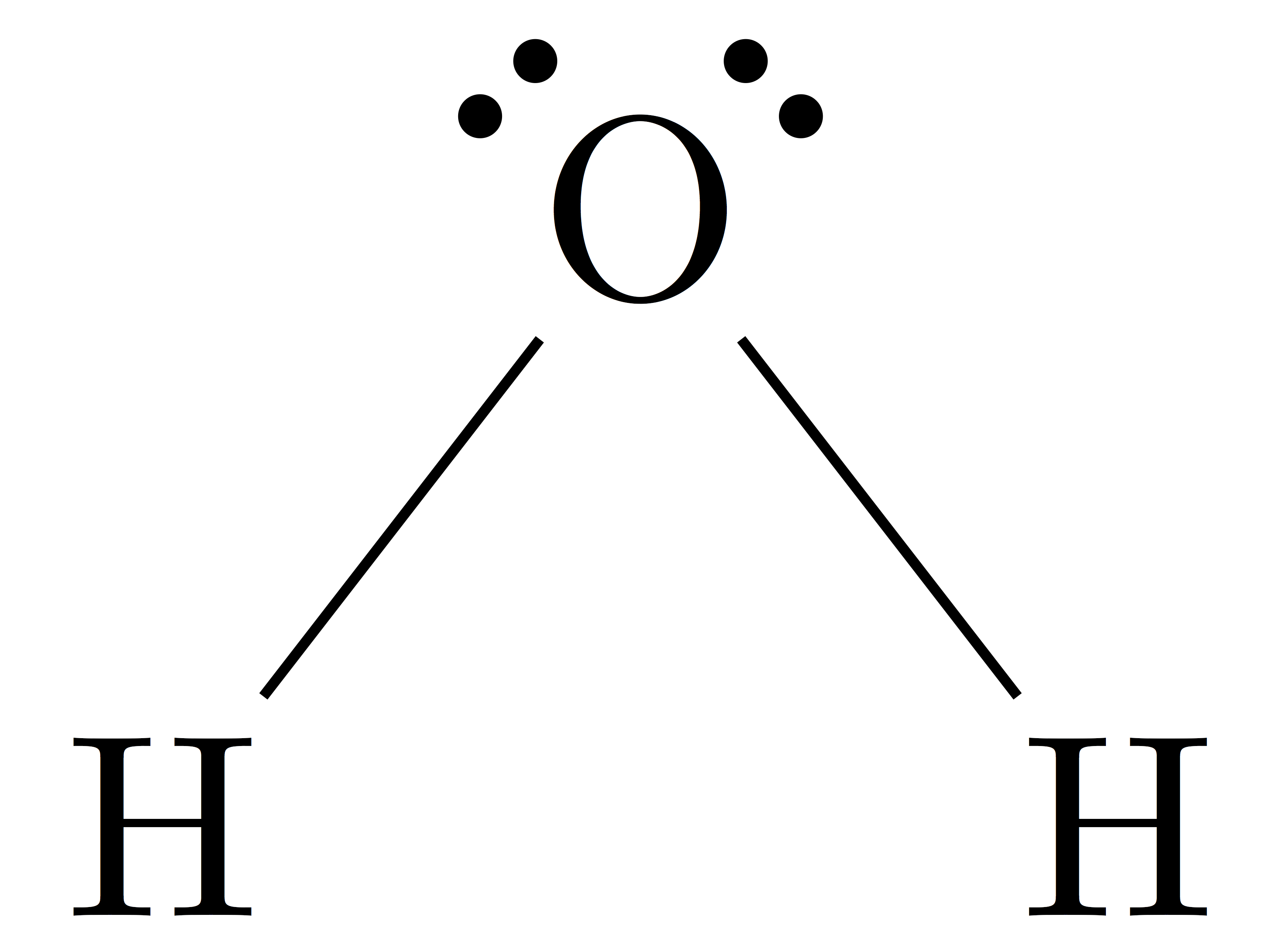
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.
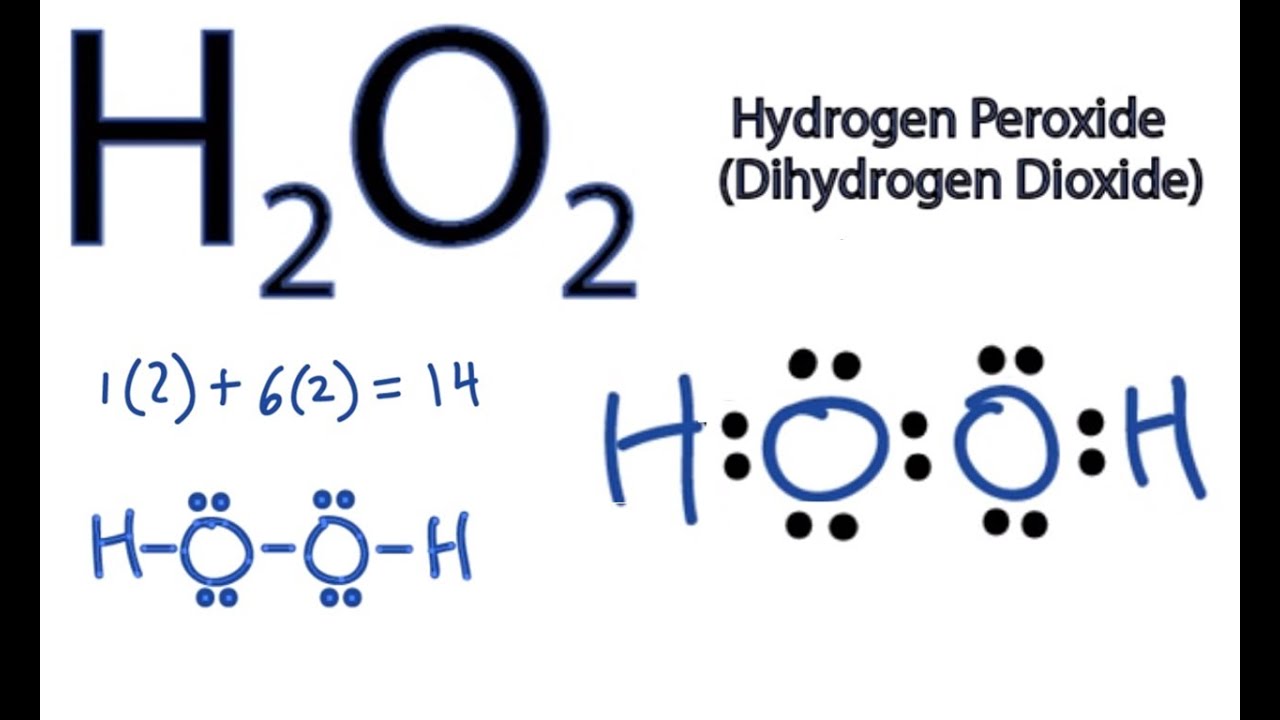
H2O2 Lewis Structure How to Draw the Dot Structure for H2O2 YouTube
Hello Guys!Hydrogen Peroxide or Dihydrogen Dioxide consists of two hydrogen atoms and two oxygen atoms. This video on H2O2 Lewis Structure will help you unde.

H2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Let's do the Lewis structure for H2O2: Hydrogen Peroxide, also called dihydrogen dioxide. On the periodic table, Hydrogen's in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2. Oxygen, group 6 or 16, we have two of those, so let's multiply that by 2 as well for a total of 14 valence electrons. Let's.
H2o Lewis Structure Shape
h2o2 lewis structure and all other facts are discussed in this article.. H 2 O 2 is the chemical formula of Hydrogen peroxide. It has a structural formula of HOOH. Its IUPAC name is dihydrogen dioxide. The simplest compound of peroxo anion is H 2 O 2 is pale blue in colour, however; In its purest form it is colourless. The probability H 2 O 2 occurring nature is pretty rare.
Draw Step By Step The Lewis Structure For Water (H2O)
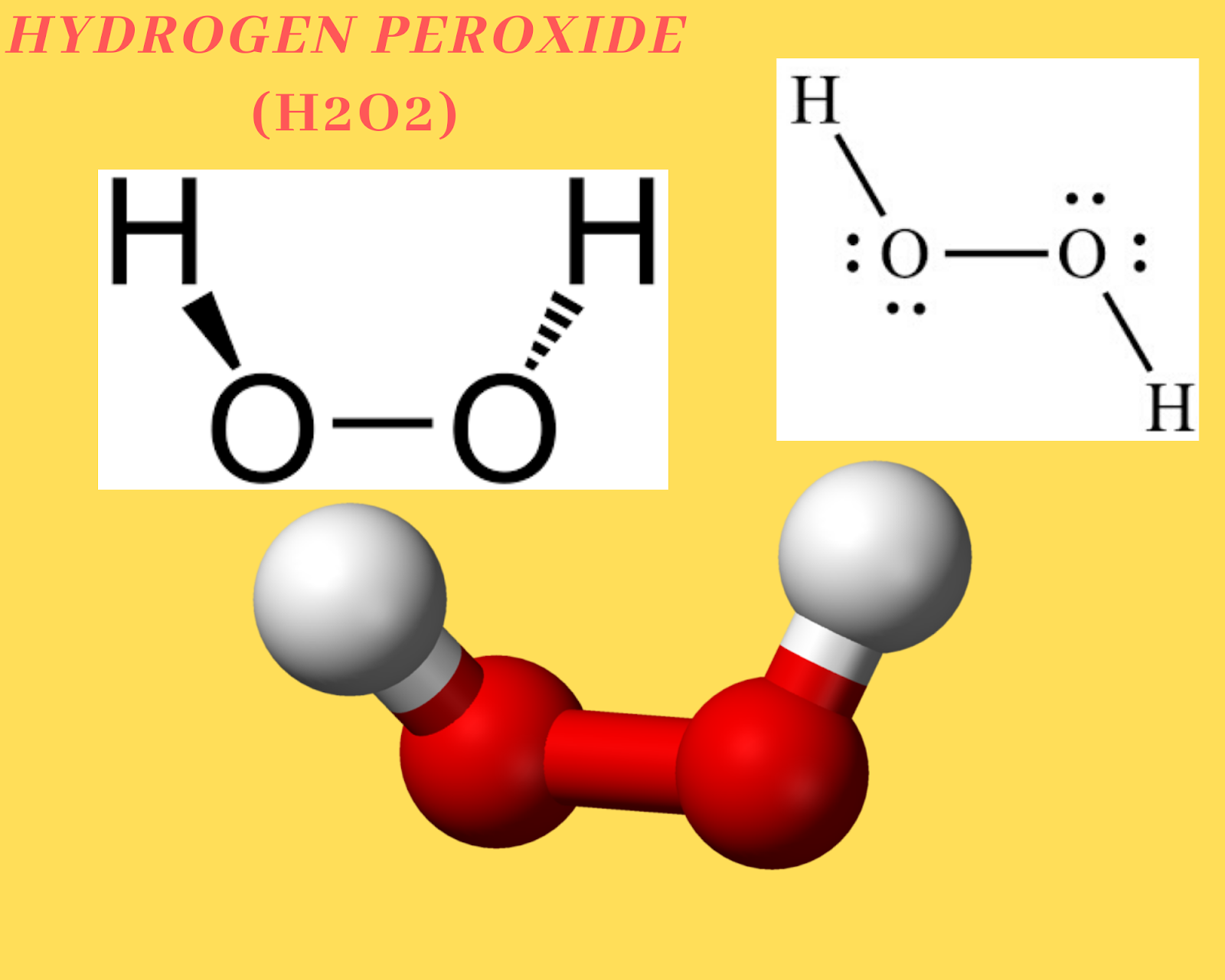
H2O2 Lewis Structure. We shall discuss the chemical bonding nature of H2O2 in this article. First, we shall draw the Lewis structure of H2O2. A Lewis structure may not give us a complete description of the molecular geometry. Still, it is helpful to visualize the overall skeletal structure of the molecule, its bonds, and its lone pairs.
Hydrogen Peroxide Properties and Uses [H2O2]
H 2 O 2 Lewis structure. H 2 O 2 (hydrogen peroxide) has two hydrogen atoms and two oxygen atoms. In the H 2 O 2 Lewis structure, there is a single bond between the two oxygen atoms, and each oxygen is attached with one hydrogen atom, and on each oxygen atom, there are two lone pairs. Steps. #1 Draw a rough skeleton structure.
Hydrogen peroxide solution 95299 Honeywell Research Chemicals
A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide).For the H2O2 structure use the periodic table to find the total nu.

Structure of h2o2? explain with diagram Brainly.in
H2O2, hydrogen peroxide is a funny looking molecule: It has two oxygen atoms in the centre, and they end up single-bonded. Then, there is a Hydrogen Atom at.

Hydrogen Peroxide Chemical Structure H2o2 Stock Vector (Royalty Free
The lewis structure of h2o2 shows that each oxygen atom is bonded to the central hydrogen atom, and each oxygen atom also has an unshared pair of electrons. This arrangement gives hydrogen peroxide its bent molecular shape. In this article, we will explore the lewis structure of h2o2 and its significance in understanding the molecule's.

Lewis Structure of H2O2, Hydrogen Peroxide YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2O2 (or hydrogen peroxide). In order to draw the lewis structure of H2O2, first of all you have to find the total number of valence electrons present in the H2O2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

Lewis Structure Hydrogen Peroxide Scientific Vector Stock Vector
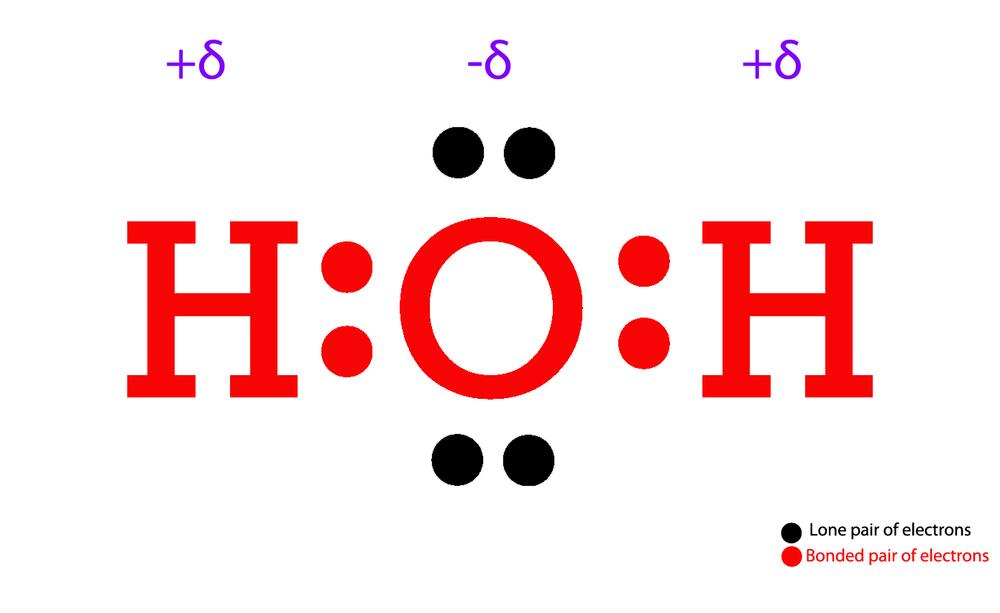
The Lewis Structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule. Because of the two lone pairs, H 2 O will have a bent molecular geometry and it will be a polar molecule. Remember that Hydrogen only needs two electrons to have a full outer shell. Video: Drawing the Lewis Structure.

Lewis Structure Hydrogen Peroxide H2o2 Scientific Stock Vector (Royalty
A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide). Note that the H2O2 Lewis structure is frequently used on tests a.
Lewis structure
To draw the Lewis structure of H2O2, we need to follow a few simple steps: Step 1: Count the total number of valence electrons in the molecule. H2O2 has 2 hydrogen atoms, 2 oxygen atoms, and 4 valence electrons (6 for oxygen and 1 for hydrogen). Therefore, the total number of valence electrons in H2O2 is 2 × 1 + 2 × 6 = 14.